Answer: The correct option is A.
Step-by-step explanation: For a radioactive isotope of potassium, which is
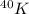 having 19 protons and 21 neutrons, the decay processes are beta-decay and electron capture.
having 19 protons and 21 neutrons, the decay processes are beta-decay and electron capture.
Potassium-40 undergoing decay processes:
- Electron Capture: In this process, the unstable nuclei becomes stable by converting electron into neutron with the help of proton.
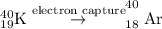
- Beta-decay: In this process, the unstable nuclei becomes stable by converting a neutron into proton and electron.
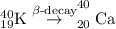
From the two processes, the K-40 isotope forms
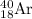 by electron capture. So, the correct option is A.
by electron capture. So, the correct option is A.