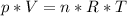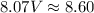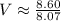Data:
p (pressure) = 81.8 kPa = 81.8*10³ Pa ≈ 8.07 atm
v (volume) = ? (in L)
n (number of mols) = 0.352 mol
R (Gas constant) = 0.082 (atm*L/mol*K)
T (temperature) = 25ºC converting to Kelvin, we have:
TK = TC + 273 → TK = 25 + 273 → TK = 298
Formula:
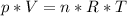
Solving: