Answer :
(1) The number of molecules of water produced are,
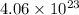
(2) The number of moles of water produced are, 45 moles
Explanation :
The balanced chemical reaction will be,
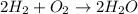
For 1 :
From the balanced reaction, we conclude that
As, 1 molecule of oxygen react to produces 2 molecules of water
So,
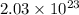 molecule of oxygen react to produces
molecule of oxygen react to produces
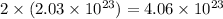 molecules of water
molecules of water
Therefore, the number of molecules of water produced are,
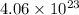
For 2 :
From the balanced reaction, we conclude that
As, 1 mole of oxygen react to produces 2 mole of water
So, 22.5 mole of oxygen react to produces
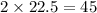 moles of water
moles of water
Therefore, the number of moles of water produced are, 45 moles