Answer : The number of moles of water produced are, 6.50 moles
Explanation : Given,
Moles of carbon dioxide
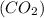 = 3.25 mole
= 3.25 mole
The given balanced chemical reaction is :
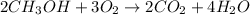
From the given balanced chemical reaction, we conclude that 2 moles of methyl alcohol react with 3 moles of oxygen to produced 2 moles of carbon dioxide and 4 moles of water.
When 2 moles of carbon dioxide is produced then 4 moles of water is also produced
Now, when 3.25 moles of carbon dioxide is produced then
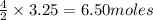 of water is also produced
of water is also produced
Therefore, the number of moles of water produced are, 6.50 moles