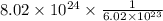Answer:
Number of moles = 13.3 moles
The appropriate ratio will be 1/6.02*10²³
Explanations:
According to the Avogadro's constant;
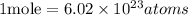
Given the following parameters
Atoms of neon = 8.02 x 10²⁴ atoms
Find the moles of neon
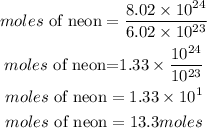
Hence the number of moles this would be is approximately 13.3 moles
The appropriate ratio will be expressed as: