Answer:
44.8L
Step-by-step explanation:
In order to get the volume of the gas that would be occupied by two moles of propane gas, we need to first calculate the mass of two moles of propane gas.
Determine the required mass of propane
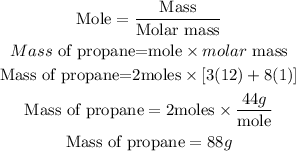
At standard temperature and pressure (STP)
44g of the gas will occupy 22.4L volume. Hence 88g of propane gas will occupy:

Therefore the volume that would be occupied by two moles of propane gas, C3H8, at standard conditions is 44.8L.