According to the given information, the reaction rate is proportional to the initial concentration of the reactants.
To compare how many times faster is reaction 2, we need to find the ratio of the initial concentrations of both reactions.
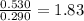
Now, let's state the rate law for both reactions:
![\begin{gathered} r1=k\lbrack A1]^2 \\ r2=k\lbrack A2]^2 \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/ir9l0y8ct0sckvcf3pf4.png)
Remember that A2=1.83A1:
![\begin{gathered} r1=k\lbrack A1]^2 \\ r2=k\lbrack1.83A1]^2=k\cdot3.35\lbrack A1]^2 \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/7fem2pinl5e9te0p8avc.png)
Find the ratio of r2 and r1:
![\begin{gathered} (r2)/(r1)=(k\cdot3.35\lbrack A]^2)/(k\cdot\lbrack A]^2) \\ (r2)/(r1)=3.35 \\ r2=3.35r1 \end{gathered}](https://img.qammunity.org/qa-images/2023/formulas/chemistry/college/rv19rv1bv16sstqcwggo.png)
It means that Reaction 2 is 3.35 times faster than Reaction 1.