Answer:
10 electrons.
Step-by-step explanation:
Hello!
In this case, since a normal atom of magnesium has 12 protons and electrons when is not in the form of a cation, since it is a metal that tends to lose electrons when ionized, and therefore it becomes positively charged, we infer that in form of:
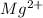
It only has 10 electrons.
Best regards!