An isotope corresponds to the variation of an element that has a different number of neutrons, that is, its mass number changes.
In this case, each color of a candium will correspond to an isotope. To find the atomic mass, we take the average of the mass pieces of the isotopes multiplied by their abundance.
We have that the average atomic mass for their candium sample will be:
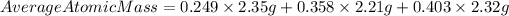
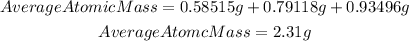
Answer: the average atomic mass for their candium sample is 2.31 g