Answer:
Molar mass of
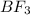 is 67.805 g.
is 67.805 g.
Step-by-step explanation:
Molar mass of a molecule is the sum of atomic masses of all constituting atoms.
In
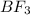 , there are one boron atom and three fluorine atoms.
, there are one boron atom and three fluorine atoms.
Atomic masses of boron and fluorine are 10.811 g and 18.998 g respectively.
So molar mass of
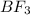 = (10.811 g)+(
= (10.811 g)+(
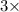 18.998 g) = 67.805 g
18.998 g) = 67.805 g