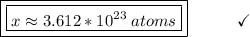Adopting the number of avogrado 6.02 * 10²³ / mol
Sodium chloride (table salt) Molar Mass = 58.44 g / mol
We will first have to find the number of moles in 35 grams of the element, like this:
1 mol ----------------- 58.44 g
X ---------------------- 35 g
58.44 * x = 35 * 1
58.44x = 35
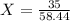
X = 0.598904...
X ≈ 0.60 mol
Now we will find how many atoms there are in 0.60 mol of this element, like this:
1 mol -------------------- 6.02 * 10²³ atoms
0.60 mol ----------------- X
X = 0.60 * 6.02 * 10²³