Answer: Option (D) is the correct answer.
Step-by-step explanation:
A reaction equation that shows (aq) along with the formula of a compound or element means that compound is present in an aqueous state. Whereas (l) means compound is present in a liquid state and (s) means that compound is present in a solid state.
For example,
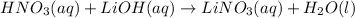
Hence, we can conclude that this given chemical reaction equation shows that aqueous solutions of nitric acid and lithium hydroxide react to produce aqueous lithium nitrate and water.