Answer: Option (4) is the correct answer.
Step-by-step explanation:
An elementary reaction is a single step reaction in which two or more chemical substances react to form products.
For example,
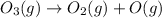 is an elementary reaction.
is an elementary reaction.
Whereas a rate determining step helps to determine the rate or speed at which overall reaction is taking place. It is the slowest step.
Complex reactions occur in more that one step, therefore, they show rate determining step.
Hence, we can conclude that the statement proceeds at the lowest rate describes the elementary reaction of a reaction mechanism that is the rate-determining step.