Answer: Option (a) is the correct answer.
Step-by-step explanation:
When an atom or element gains an electron then the atom acquires a negative charge whereas when an atom or element loses an electron then it acquires a positive charge.
for example, atomic number of fluorine is 9 and when it gains one electron then fluorine atom acquires a negative charge. Hence, neutral atom of fluorine changes into
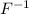 ion.
ion.
Thus, we can conclude that
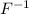 ion is formed when an atom of fluorine (F) gains one electron.
ion is formed when an atom of fluorine (F) gains one electron.