Answer: E. trigonal planar
Explanation:
According to VSEPR theory:
![:{\text{Number of electrons}} =(1)/(2)[V+N-C+A]](https://img.qammunity.org/2018/formulas/chemistry/high-school/zv6bipyuyp9vnqs91bvuy2bi48bxm13jt8.png)
where, V = number of valence electrons present in central atom i.e. carbon = 4
N = number of monovalent atoms bonded to central atom=3
C = charge of cation = 0
A = charge of anion = -1
If a central atom is bound to three other atoms with no lone pairs of electrons,the number of electrons is 3 that means the hybridization will be
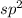 and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.
and the electronic geometry of the molecule will be trigonal planar as the electron pairs will repel each other and attain a position which is most stable.