Answer:
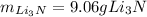
Step-by-step explanation:
Hello!
In this case, since the reaction between nitrogen and lithium to obtain lithium nitride is:
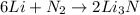
Starting by that amount of atoms of lithium, we first need to compute the moles of lithium via the Avogadro's number:
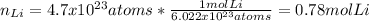
Now, by using the 6:2 mole ratio between lithium and lithium nitride and the molar mass of this product (34.83 g/mol), we can compute the required grams as shown below:
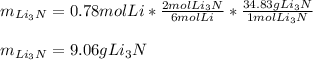
Best regards!