Answer:
C. 0.04 moles per cubic decimeter.
Step-by-step explanation:
The molar mass of the Iodine is 253.809 grams per mole and a cubic decimeter equals 1000 cubic centimeters. The concentration of Iodine (
 ), measured in moles per cubic decimeter, can be determined by the following formula:
), measured in moles per cubic decimeter, can be determined by the following formula:
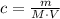 (1)
(1)
Where:
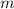 - Mass of iodine, measured in grams.
- Mass of iodine, measured in grams.
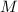 - Molar mass of iodine, measured in grams per mol.
- Molar mass of iodine, measured in grams per mol.
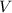 - Volume of solution, measured in cubic decimeters.
- Volume of solution, measured in cubic decimeters.
If we know that
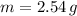 ,
,
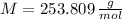 and
and
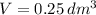 , then the concentration of iodine in a solution is:
, then the concentration of iodine in a solution is:
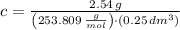
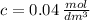
Hence, the correct answer is C.