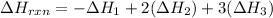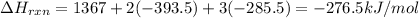Answer:
The change in enthalpy for the formation of ethanol is -276.5 kJ/mol
Step-by-step explanation:
The given reactions are:
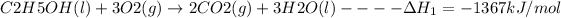


The required reaction involves the formation of C2H5OH from C, H2 and O2:
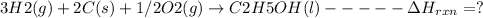
This can be obtained by reversing reaction (1), multiplying equation (2) by 2, multiplying equation (3) by 3 and then adding the corresponding ΔH values