Explanations:- Acetate ion is a conjugate base of a weak acid(acetic acid). From Bronsted-Lowry concept of acid and base, "Acids are proton donor where as bases are proton acceptors.
To work as a base, acetate ion would be accepting a proton. Water is an amphoteric compound means it could act as an acid as well as a base. So, let's write the reaction of acetate ion with water to show how it acts as a base.
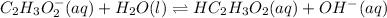
In the above reaction, acetate ion has accepted a proton from water means its a base and since water has donated a proton so its an acid.
Acetic acid is the conjugate acid of acetate ion and hydroxide ion is the conjugate base of water.