Answer : (1) The number of molecules will be,
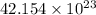
(2) The number of moles of chlorine atoms is, 6.0112 moles
Solution :
(1) As, 1 mole
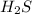 contains
contains
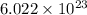 number of molecules
number of molecules
So, 7 moles
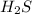 contains
contains
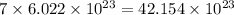 number of molecules
number of molecules
Therefore, the number of molecules will be,
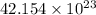
(2) Number of atoms of magnesium chloride =

In one magnesium chloride molecule there are two chlorine atoms.
Number of chlorine atoms in
 atoms of magnesium chloride =
atoms of magnesium chloride =
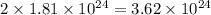
1 mole =
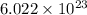 atoms
atoms
Moles of chlorine =
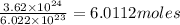
Therefore, the number of moles of chlorine atoms is, 6.0112 moles