Data:
V (volume) = 500.0 mL = 0.5 L
T (temperature) = 15.00ºC
(converting in Kelvin) → TK = TC + 273 → TK = 15 + 273 = 288 K
P (pressure) = 736.0 mmHg
R (constant) = 62.363 (mmHg*L/mol*K)
m (mass) = 2.688 g
M (Molar Mass) = ? (g/mol)
Formula: General Gas Equation
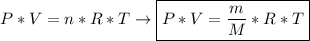
Solving:
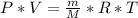
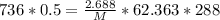
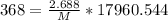
Product of extremes equals product of means:
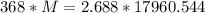
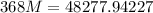
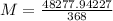
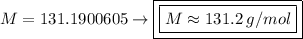
Therefore:
The gas found to have such a molar mass is xenon gas