The pH of the calculated solution is 9.17 this tells us that it is a base
The calculation of the pH
The conjugate acid = 1.8x10⁻⁵
pH = 0.400
We have NaCH3CO2 solution and CH3CO2H
We first have to find kb given that we have ka
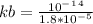
= 5.6x10⁻¹⁰
5.6x10⁻¹⁰ = x*x/0.4
Cross multiply
2.2x10⁻¹⁰ = x²
x = 1.5x10⁻⁵
Take the antilog of this
= 4.83
POH + pH = 14
pH = 14-4.83
= 9.17
This shows that the substance is a base.