First, we write out a balanced equation.
HA <--> H(+) + A(-)
Next, we create an ICE table
HA <--> H+ + A-
[]i 0.40M 0M 0M
Δ[] -x +x +x
[]f 0.40-x x x
Next, we write out the Ka expression.
Ka = [H+][A-]/[HA]
Ka = x*x/(0.40-x)
However, because Ka is less than 10^-3, we can assume the amount of dissociation is negligible. Thus,
Assume 0.40-x ≈ 0.40
Therefore, 1.2x10^-6 = x^2/0.40
Then we solve for the [H+] concentration, or x
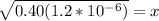
x=6.93x10^-4
Next, to find pH we do
pH = -log[H+]
pH = -log[6.93x10^-4]
pH = 3.2