Answer: The moles of hydrogen in given moles of caffeine are 28 moles.
Step-by-step explanation:
We are given:
Moles of caffeine = 2.8 moles
The chemical formula of caffeine is
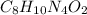
In 1 mole of caffeine, 8 moles of carbon atoms, 10 moles of hydrogen atoms, 4 moles of nitrogen atoms and 2 moles of oxygen atoms are present.
So, in 2.8 moles of caffeine, moles of hydrogen atoms present will be
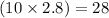 moles
moles
Hence, the moles of hydrogen in given moles of caffeine are 28 moles.