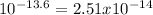Answer:
2.51 x 10-14 M
Step-by-step explanation:
The definition of pOH is:
pOH = -log[OH-]
then, clearing of the equation [OH-] we have:
-pOH = log[OH-]
By properties of logarithms:
![[OH-] = 10^(-pOH)](https://img.qammunity.org/2018/formulas/chemistry/high-school/61qttznoqlnuggcihm9l7d2dy7sca9elpa.png)
Replacing the values:
![[OH-] = 10^(-13.6)](https://img.qammunity.org/2018/formulas/chemistry/high-school/inofmfz4356nls42swkfcqq62jlqwi1fxm.png)
And if you use a calculator, you will see that