Answer: The pH of the solution is 1.49
Step-by-step explanation:
pH is defined as the negative logarithm of hydrogen ion concentration present in a solution.
To calculate the pH of the reaction, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9so9u3mmeurzbcqkpf3jt5b89ctmc03tdp.png)
where,
![[H^+]=3.26* 10^(-2)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/vr07zfmsnn6d2lkjvc8jscmpeznpwe76zq.png)
Putting values in above equation, we get:
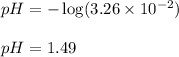
Hence, the pH of the solution is 1.49