Answer: The pH of the solution is 1.92
Step-by-step explanation:
To calculate the pH of the solution, we use the equation:
![pH=-\log[H^+]](https://img.qammunity.org/2018/formulas/chemistry/high-school/9so9u3mmeurzbcqkpf3jt5b89ctmc03tdp.png)
We are given:
Hydrochloric acid is a monoprotic acid and it dissociates 1 mole of hydrogen ions. So, the concentration of hydrogen ions is
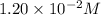
![[H^+]=1.20* 10^(-2)M](https://img.qammunity.org/2018/formulas/chemistry/high-school/j62m8a6i3rzrh1xctkkj9by7gy4bk96slh.png)
Putting values in above equation, we get:
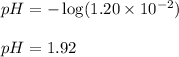
Hence, the pH of the solution is 1.92