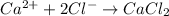Answer:
The compound represents that calcium chloride is an ionic salt made up of 1 calcium ion and 2 chloride ions.
Step-by-step explanation:
The given chemical formula
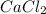 is of calcium chloride salt.
is of calcium chloride salt.
The chemical formula represents that 1 atom calcium binds with two atoms of chlorine to form a single molecule of calcium chloride.
It is an ionic salt made up 1 calcium ion and 2 chloride ions.
Calcium has atomic number of 20 and chlorine has atomic number of 17. Calcium will loose 2 electrons to gain noble gas stability whereas chlorine atom will gain 1 electron to gain noble gas stability.
![[Ca]=[Ar]4s^2](https://img.qammunity.org/2018/formulas/chemistry/high-school/pchv4ltjpep27f1x2skl52in0nyst70xn9.png)
![[Ca^(2+)]=1s^22s^2p^63s^23p^6](https://img.qammunity.org/2018/formulas/chemistry/high-school/wbnwwdjq6z2371e77up150u6q9t597jrdz.png)
![[Cl]=1s^22s^2p^63s^23p^5](https://img.qammunity.org/2018/formulas/chemistry/high-school/xk2gkqhiu52u4en8x1v8ct5krox6wyyx8z.png)
![[Cl^(-)]=1s^22s^2p^63s^23p^6](https://img.qammunity.org/2018/formulas/chemistry/high-school/18a12fd2pgvlr2ldm279vpt4as8ooba40q.png)