percent by volume is a way to measure concentration %V and it is calculated as the volume of the solute (Vs) , in this case ethanol by the total volume of the solution (Vt) and multiplied by 100:
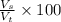
We have the volume of the solute 95 ml and the total volume of the solution 1L so We only have to divide one by the other, just remember that they need to be in the same units:
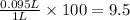
The answer is %V=9.5%