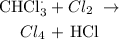Answer:
Step-by-step explanation:
a) Here, we want to get the mechanism of the reaction between chlorine and methane in the presence of ultraviolet light
The first thing that happens here is called radicalization. It simply involves the attack of a chlorine gas molecule by the ultraviolet rays of the sun to produce two chlorine radicals that are ready to attack the methane molecule. This is known as the initiation stage
We have this as:
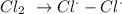
This radical then attack the methane molecule to give a CH3 radical alongside Hydrogen chloride
We have that as:
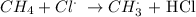
The CH3 radical then attacks a chlorine gas molecule to give the chloromethane alongside another chlorine radical:
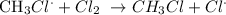
This is known as the propagation stage
For the termination stage, a CH3 radical can react with free chlorine radical as shown below:

b) i) To produce dichloromethane, a chlorine molecule will be attacked by chloromethane radical:
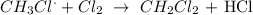
II) For ethane to be formed, two methyl radicals will combine to give an ethane molecule as shown below:

iii) Tetrachloromethane can be formed when all the hydrogen atoms in methane are replaced by chlorine atoms by a looping activity of propagation and termination
Basically, it happens when a trichloromethane radical attacks a chlorine gas molecule