Hello!
if a gas is cooled from 323.0 K to 273.15 k and the volume is kept constant what final pressure would result if the original pressure was 750.0 mmHg
We have the following information:
P1 (initial pressure) = 750.0 mmHg
T1 (initial temperature) = 323.0 K
P2 (final pressure) = ? (in mmHg)
T2 (final temperature) = 273.15 K
According to the Law of Charles and Gay-Lussac in the study of gases, we have an isochoric (or isovolumetric) transformation when its volume remains constant or equal, then we will have the following formula:
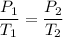
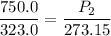
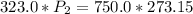

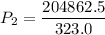
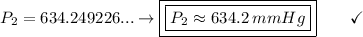
_______________________________
I Hope this helps, greetings ... Dexteright02! =)