Answer: The amount of energy required to raise the temperature is 13323.75 joules.
Explanation :
The amount of energy required to raise the temperature can be calculated as follows.
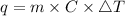
where,
q = heat energy
m = mass of water
C = specific heat
T = temperature
Remember that the specific heat of water is
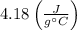 .
.
Therefore, putting the values in the above equation as follows.
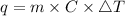
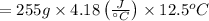
= 13323.75 joules
So, the amount of energy required to raise the temperature is 13323.75 joules.