Answer:
3 half-lives have passed.
The bones are 17 190 years old.
Step-by-step explanation:
The half-life of a radioactive element is the time required for the substance to lose half of its initial activity. In other words, the half-life is denoted by this symbol
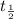
Let's take the half-life of carbon as
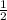
After a certain number of years, the activity degenerates to half of the original activity. So it will be
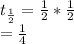
After some years, the activity will be
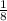
Thus three half-lives have passed. A simple formula to remember the pattern is to use the exponential expression :
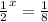
solving for x gives x = 3
hence there half-lives.
For carbon,
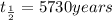
since there half-lives have passed, the age of the sample will be
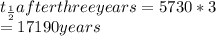
The sample will be 17 190 years.