Step-by-step explanation:
Whenever an electron jumps from higher energy state to lower energy state it emit out energy in the form of electromagnetic radiation.
The amount of energy liberated or emitted after the transition is determined by the help of Rydberg's Equation:
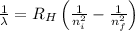
Where,
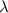 = Wavelength of radiation
= Wavelength of radiation
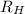 = Rydberg's Constant
= Rydberg's Constant
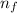 = Higher energy level =
= Higher energy level =
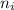 = Lower energy level
= Lower energy level
Energy is related to wavelength that is Planck's equation:
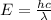
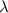 = Wavelength of radiation
= Wavelength of radiation
E= energy emitted by the electron on transition