The molecular formula : As₄S₆
Further explanation
Given
Rate of effusion of arsenic(III) sulfide = 0.28 times the rate of effusion of Ar atoms
Required
The molecular formula
Solution
Graham's law: the rate of effusion of a gas is inversely proportional to the square root of its molar masses or
the effusion rates of two gases = the square root of the inverse of their molar masses:
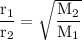
or
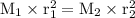
Input the value :
1 = Arsenic(III) sulfide
2 = Ar
MM Ar = 40 g/mol
0.28 = √(40/M₁)
M₁=40 : 0.28²
M₁=510 g/mol
The empirical formula of arsenic(III) sulfide = As₂S₃
(Empirical formula)n = molecular formula
( As₂S₃)n = 510 g/mol
(246.02 g/mol)n = 510 g/mol
n = 2
So the molecular formula : As₄S₆