Answer:
The number of years is approximately 23 years.
Explanation:
Given : A certain isotope decays so that the amount A remaining after t years is given by :
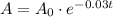 where
where
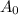 is the original amount of the isotope.
is the original amount of the isotope.
To find : To the nearest year, the half-life of the isotope (the amount of time it takes to decay to half the original amount) is how many years?
Solution :
The decay model is given by
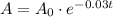
We have given that,
The amount of time it takes to decay to half the original amount.
i.e.
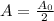
Substitute the values in the formula,
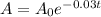
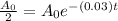
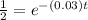
Taking natural log both side,
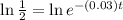
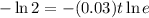
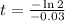
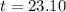
Therefore, The number of years is approximately 23 years.