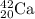Answer:
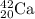
Step-by-step explanation:
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
Given mass number of calcium= 42
Atomic number is defined as the number of protons or number of electrons that are present in an atom.
Atomic number = Number of electrons = Number of protons
General representation of an element is given as:
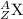
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
Calcium is the 20th element of the periodic table with atomic number of 20 and is written as