Answer: The concentration of
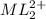 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.
Step-by-step explanation:
We are given:
![[M^(2+)]_(initial)=0.100M](https://img.qammunity.org/2018/formulas/chemistry/college/30ub21anikipsipok1mayuxscutkl4dpg9.png)
![[L]_(initial)=0.100M](https://img.qammunity.org/2018/formulas/chemistry/college/p30uft1qxercbgpp28b7r8jxnc1oe7kyrx.png)
For the given chemical equation:
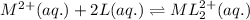
Initially: 0.100M 0.100M
At eqllm: 0.100 - x 0.100 - 2x x
We are also given:
![[L]_(eqllm)=0.0100M](https://img.qammunity.org/2018/formulas/chemistry/college/d38axelweovyvm28l55y7911wdhf2zpu24.png)
Equating the two values:
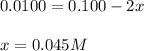
Hence, the concentration of
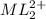 at equilibrium is 0.045 M.
at equilibrium is 0.045 M.