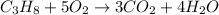Answer: Decomposition
Explanation:
1. Synthesis is a chemical reaction in which two or more reactants combine to combine to give a single product.
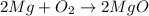
2. Decomposition reaction is a chemical reaction in which one reactant gives two or more than two products on absorption of energy.
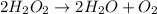
3.. Combustion is a chemical reaction in which hydrocarbon burns in the the presence of oxygen to form carbon dioxide, water and energy.