Answer:
0.14 moles of water are produced.
Step-by-step explanation:
1st) From the balanced reaction we know that 1 mole of Fe2O3 reacts with 3 moles of H2 to produce 2 moles of Fe and 3 moles of H2O. Using the molar mass of Fe2O3 (160g/mol) and H2 (2g/mol) we can convert the moles to grams:
- Fe2O3 conversion: it is not necessary to calculate the conversion, because 1 mole is equal to 160g.
- H2 conversion:
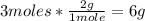
So, from the balanced reaction, 160g of Fe2O3 react with 6g oh H2.
2nd) Now we have to find out which compound is the limiting reactant and which compound is the excess reactant, using the 7.3g and 15.3g from the exercise:
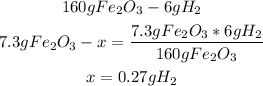
Now we know that the 7.3g of Fe2O3 will need 0.27g of H2 to react properly, but in this case we have 15.3g of H2, so H2 is the excess reactant and Fe2O3 is the limiting reactant.
3rd) Finally, we can calulate the moles of water that are produced using the grams of the limiting reactant:
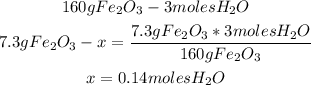
So, 0.14 moles of water are produced.