Answer:
The Fe increases its oxidation number from 0 to +2, it means that electrons are lost, therefore the Fe is the reducing agent and is the oxidized substance.
Step-by-step explanation:
1. First write down the chemical equation:
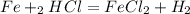
2. Then determine the oxidation numbers for each compound:
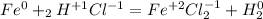
3. Write the elements that change its oxidation number:
-
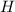
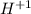 →
→
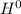
The H decreases its oxidation number from +1 to 0, it means that electrons are gained, therefore the H is the oxidizing agent and is the reduced substance.
-
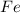
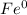 →
→
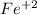
The Fe increases its oxidation number from 0 to +2, it means that electrons are lost, therefore the Fe is the reducing agent and is the oxidized substance.