Answer:- D. 0.20 m
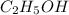
Explanations:- When a on volatile solute is added to a solvent then the boiling point of the solution increases(elevated) and we call it elevation in boiling point.
Elevation in boiling point is directly proportional to the molality of the solution and the Van't Hoff factor "i" which is the number of ions we get on dissociation.
The equation is written as:

where
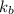 is the boiling point elevation constant.
is the boiling point elevation constant.
We have all the aqueous solutions, so the boiling point elevation constant value will remain same for all of them. The molality for all the solutions is also same. So, elevation in boiling point will depend only on the value of Van't Hoff factor.
Lower is the value of "i", lower will be the boiling point and vice versa.
The question asks, which aqueous solution has the lowest boiling point. So, we nede to choose the one with the lowest value of "i".
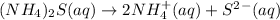
i = 3
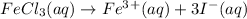
i = 4
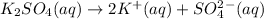
i = 3
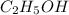 is a covalent molecule and so it does not dissociate to give the ions and hence the value of "i" for this is 1.
is a covalent molecule and so it does not dissociate to give the ions and hence the value of "i" for this is 1.
Comparing the "i" values for all the solutions, the least value is for ethyl alcohol means the boiling point of 0.20 m ethyl alcohol solution will be lowest.
So, the correct answer is D. 0.20 m
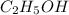 .
.