We are asked to predict a reaction having two solids as reactants. The reaction occurs in the solid phase. Initially the elements are in their natural state, so they will have an oxidation state equal to 0.
This is a combination reaction, so the product will be a combination of aluminum and sulfur.
When combined, aluminum has an oxidation state of +3, this means that it gives up its electrons. Sulfur has an oxidation state of -2. The resulting product will be aluminum sulfide (Al2O3). so, the reaction will be:
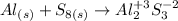
We must balance the equation.
Let's start with sulfur. We multiply each sulfur-containing molecule by its opposite. In other words, we multiply the S8 molecule by 3 and we multiply the S3 molecule by 8.
And to balance the aluminum we must multiply the aluminum molecule by 16. So, the balanced equation will be:
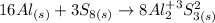
So, the answer, in summary, will be:
We obtain a product with the formula Al2O3. Al is an ion with a charge of +3, and S is an ion with a charge of -2.