Answer: The correct answer is Option B.
Step-by-step explanation:
Polymerization reaction is defined as a reaction in which monomer molecules react together to form a polymer chains which are three-dimensional networks.
Synthesis reaction is defined as a chemical reaction in which two or more chemical species react in their naturally occurring state to form a single large compound.
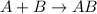
Replacement reaction is defined as a chemical reaction in which exchange of ions takes place. They are of two types: Single replacement and double replacement.
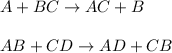
Decomposition reaction is a type of chemical reaction in which a larger molecule breaks down into two or more smaller molecules.
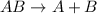
The given reaction follows:
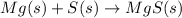
This is a type of synthesis reaction.
Hence, the correct answer is Option B.