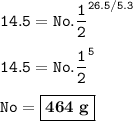464 g radioisotope was present when the sample was put in storage
Further explanation
Given
Sample waste of Co-60 = 14.5 g
26.5 years in storage
Required
Initial sample
Solution
General formulas used in decay:
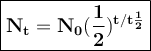
t = duration of decay
t 1/2 = half-life
N₀ = the number of initial radioactive atoms
Nt = the number of radioactive atoms left after decaying during T time
Half-life of Co-60 = 5.3 years
Input the value :