Answer: 1.38 moles
Explanation: According to Avogadro's law, 1 mole of every substance contains Avogadro number of particles (atoms, molecules or ions) i.e.
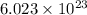 particles.
particles.
Given : 1 mole molecule of water contains
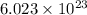 molecules of water.
molecules of water.
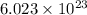 molecules are present in = 1 mole
molecules are present in = 1 mole
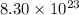 molecules will be present in =
molecules will be present in =
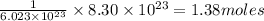
Thus
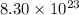 molecules is equal to 1.38 moles.
molecules is equal to 1.38 moles.