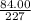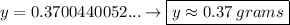Molar Mass of Nitroglycerin
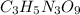
C = 3*12 = 36 amu
H = 5*1 = 5 amu
N = 3*14 = 42 amu
O = 9*16 = 144 amu
----------------------------
Molar Mass of
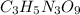
= 36+5+42+144 = 227 g/mol
Molar mass of all nitrogen = 42 g/mol
Solving: Rule of three (directly proportional)
2.00 grams ----------------- 227 g/mol
y grams --------------------- 42 g/mol
Product of extremes equals product of means:
227*y = 2.00*42
227y = 84.00
y =