Answer : The correct option is, (4) 50 mole
Solution : Given,
Moles of NO = 40 mole
The given balanced chemical reaction is,
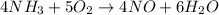
As, 4 moles of NO produces from 5 moles of

So, 40 moles of NO produces from
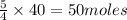 of
of

Therefore, the total number of moles of
 is, 50 moles
is, 50 moles