Explanation :
According to the first law of thermodynamic, the energy cab be transformed from one form of energy to the another form of energy but the energy can neither be created nor be destroyed. It is also called as the law of conservation of energy.
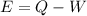
where,
E = internal energy
Q = net heat transferred into the system
W = work done by the system or on the system
So, we can say that the heat that flows into the system is transformed into work and a change in internal energy.