Answer:
Isotopes of an element have same number of protons but different number of neutrons. Which means isotopes of an element have same atomic number but different mass number.
The chemical property of an element is determined by the number of electrons. And as all the isotopes have same number of electrons, they have same chemical properties.
Thus as isotopes of an element have same atomic number , they have same number of electrons and protons. As they have different mass number, the number of neutrons will be different. Hydrogen has three isotopes ,
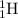 ,
,
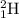 and
and
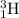 . Thus
. Thus
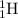 has no neutron.
has no neutron.